Difference between revisions of "PrePex"
(Created page with "{{Construction Site}} thumb|Single-use PrePex Device. thumb|Single-use Sizing Plate. The PrePex is a circumcision de...") |
m |
||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
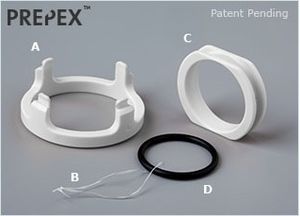
[[File:Prepex-parts.jpg|thumb|Single-use PrePex Device.]] [[File:Prepex-sizing.jpg|thumb|Single-use Sizing Plate.]] | [[File:Prepex-parts.jpg|thumb|Single-use PrePex Device.]] [[File:Prepex-sizing.jpg|thumb|Single-use Sizing Plate.]] | ||
| − | The | + | The '''PrePex''' is a circumcision device which is currently marketed by Circ MedTech, Ltd., exploiting the [[WHO]]'s endorsement of circumcision as an HIV prevention method.<ref>{{REFweb |
| − | {{ | + | | last=Scientific American |
| − | | url = http://www.scientificamerican.com/article.cfm?id=rwanda-investigating-adult-male-circumcision-without-anesthesia | + | | first= |
| − | | | + | | year= |
| − | | accessdate = 2011-08-30 | + | | title=Rwanda Investigating Adult Male Circumcision sans Anesthesia |
| − | + | | url=http://www.scientificamerican.com/article.cfm?id=rwanda-investigating-adult-male-circumcision-without-anesthesia | |
| − | + | | publisher=Nature America, Inc. | |
| − | }} | + | | accessdate=2011-08-30 |
| − | </ref> It works by effecting ischemic necrosis, forcing the foreskin tissue to die and fall off. This is marketed as an "advantage" over other circumcision devices and techniques, because it makes circumcision "bloodless, simple," and the manufacturing of easily-mass-produced parts allows for "scalable male circumcision programs in resource limited settings."<ref> | + | }}</ref> It works by effecting ischemic necrosis, forcing the foreskin tissue to die and fall off. This is marketed as an "advantage" over other circumcision devices and techniques, because it makes circumcision "bloodless, simple," and the manufacturing of easily-mass-produced parts allows for "scalable male circumcision programs in resource limited settings."<ref>{{REFweb |
| − | {{ | + | | last= |
| − | | url = http://www.prepex.com/Scientific.aspx | + | | first= |
| − | | | + | | year= |
| − | | accessdate = 2011-08-30 | + | | title=Safety and efficacy validated. Device approved for use in Rwanda |
| − | + | | url=http://www.prepex.com/Scientific.aspx | |
| − | }} | + | | publisher=PrePex |
| − | </ref> In actuality, the concept of cutting off circulation to the foreskin in order to effect ischemic necrosis is found in other devices, such as the [[PlastiBell]], [[TaraKlamp]] and [[Sunathrone]] devices. | + | | accessdate=2011-08-30 |
| + | }}</ref> In actuality, the concept of cutting off circulation to the foreskin in order to effect ischemic necrosis is found in other devices, such as the [[PlastiBell]], [[TaraKlamp]] and [[Sunathrone]] devices. | ||
== Inventors == | == Inventors == | ||
| − | + | '''PrePex''' was invented by Israeli researchers Oren Fuerst, Ido Kilemnick, and Shaul Shohat. PrePex's CEO is [[Tzameret Fuerst]]. The '''PrePex''' patent application, "Circumcision Device and Method for Mass Circumcision," WIPO Patent Application WO/2011/007358, was submitted by Circ MedTech, Ltd, the company that currently markets the '''PrePex''' System.<ref>{{REFweb | |
| − | {{ | + | | last= |
| − | | url = http://www.wipo.int/patentscope/search/en/WO2011007358 | + | | first= |
| − | | | + | | year= |
| − | | accessdate = 2011-08-30 | + | | title=Circumcision device and method for mass circumcision |
| − | + | | url=http://www.wipo.int/patentscope/search/en/WO2011007358 | |
| − | }} | + | | publisher=WIPO |
| − | </ref> | + | | accessdate=2011-08-30 |
| + | }}</ref> | ||
| + | == Promotion of Male Circumcision for HIV Prevention == | ||
| + | PrePex was set to be introduced to Zimbabwe to aid their mass circumcision campaign.<ref>{{REFweb | ||
| + | | last= | ||
| + | | first= | ||
| + | | year= | ||
| + | | title= Controversial UN program brings circumcision device to Zimbabwe | ||
| + | | url=http://www.zimeye.org/?p=51308 | ||
| + | | publisher= | ||
| + | | accessdate=2012-06-21 | ||
| + | }}</ref> | ||
| − | + | PlusNews reported on a study of the PrePex device, saying "a study of 40 men in Rwanda found one adverse event at removal of the device and a healing time of 17 days".<ref>{{REFweb | |
| − | PrePex | + | | last= |
| − | {{ | + | | first= |
| − | | url = http://www. | + | | year= |
| − | | | + | | title=HIV/AIDS: Adult male circumcision – new developments |
| − | | accessdate = 2012-06- | + | | url=http://www.plusnews.org/Report/93311/HIV-AIDS-Adult-male-circumcision-new-developments |
| − | }} | + | | publisher= |
| − | </ref> | + | | accessdate=2012-06-20 |
| + | }}</ref> | ||
| − | + | Circ MedTech promotes the '''PrePex''' System as a means to "to dramatically reduce the HIV infection rate." Built in to the patent application is a statement touting male circumcision as an HIV prevention method.<ref>{{REFweb | |
| − | {{ | + | | last= |
| − | | url = http://www. | + | | first= |
| − | | | + | | year= |
| − | | accessdate = | + | | title=Why Male Circumcision? |
| − | }} | + | | url=http://www.prepex.com/Why.aspx |
| − | </ref> | + | | publisher=PrePex |
| + | | accessdate=2011-08-30 | ||
| + | }}</ref> | ||
| − | + | == Use in Africa == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | The PrePex device will be tested in Africa starting 2013. The [[President's Emergency Plan for AIDS Relief]] will pay for PrePex circumcisions for about 2,500 men in Lesotho, Malawi, South Africa, Tanzania and Uganda. The [[Bill & Melinda Gates Foundation]] will pay for similar studies in Kenya, Mozambique, South Africa, Zambia and Zimbabwe. <ref>{{REFweb |
| + | | last= | ||
| + | | first= | ||
| + | | year=2012-08-13 | ||
| + | | title=Africa: Nonsurgical Circumcision Device Will Be Tested to Help Curb AIDS | ||
| + | | url=http://www.nytimes.com/2012/08/14/health/nonsurgical-circumcision-device-will-be-tested-to-help-curb-aids.html?_r=0 | ||
| + | | publisher=The New York Times | ||
| + | | accessdate=2012-12-26 | ||
| + | }}</ref> The [[Rakai Health Sciences Program]] is currently unveiling it and testing it in Uganda, presented by Godfrey Kigozi, program chief investigator, who stated that "it was invented in the United States of America. We have so far tested it on about 50 clients but we need 250 more to confirm its acceptability but so far it has worked properly with those who used it"<ref>{{REFweb | ||
| + | | last= | ||
| + | | first= | ||
| + | | year=2012-12-26 | ||
| + | | title=New safe male circumcision method unveile | ||
| + | | url=http://www.newvision.co.ug/news/638450-new-safe-male-circumcision-method-unveiled.html | ||
| + | | publisher=New Vision | ||
| + | | accessdate=2012-12-26 | ||
| + | }}</ref>. The PrePex was not invented in the United States<ref>{{REFweb | ||
| + | | last= | ||
| + | | first= | ||
| + | | year= | ||
| + | | title=Circumcision device and method for mass circumcision | ||
| + | | url=http://www.wipo.int/patentscope/search/en/WO2011007358 | ||
| + | | publisher=WIPO | ||
| + | | accessdate=2011-08-30 | ||
| + | }}</ref>. | ||
| − | + | {{LINKS}} | |
| − | {{ | + | * [http://PrePex.com/ PrePex™ website] |
| − | + | * [http://c-medtech.com/ Circ MedTech] | |
| − | + | * [http://www.sumobrain.com/patents/wipo/Circumcision-device-method-mass/WO2011007358A2.html PrePex patent] | |
| − | + | * [http://www.nytimes.com/2012/01/31/health/aids-prevention-inspires-ways-to-simplify-circumcision.html?pagewanted=1&_r=2 AIDS Prevention inspires ways to make circumcision easier] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | {{REF}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Circumcision Products]] | [[Category:Circumcision Products]] | ||
| Line 100: | Line 103: | ||
[[Category:CircLeaks]] | [[Category:CircLeaks]] | ||
| − | |||
[[Category:From IntactWiki]] | [[Category:From IntactWiki]] | ||
Revision as of 14:48, 18 August 2019
The PrePex is a circumcision device which is currently marketed by Circ MedTech, Ltd., exploiting the WHO's endorsement of circumcision as an HIV prevention method.[1] It works by effecting ischemic necrosis, forcing the foreskin tissue to die and fall off. This is marketed as an "advantage" over other circumcision devices and techniques, because it makes circumcision "bloodless, simple," and the manufacturing of easily-mass-produced parts allows for "scalable male circumcision programs in resource limited settings."[2] In actuality, the concept of cutting off circulation to the foreskin in order to effect ischemic necrosis is found in other devices, such as the PlastiBell, TaraKlamp and Sunathrone devices.
Contents
Inventors
PrePex was invented by Israeli researchers Oren Fuerst, Ido Kilemnick, and Shaul Shohat. PrePex's CEO is Tzameret Fuerst. The PrePex patent application, "Circumcision Device and Method for Mass Circumcision," WIPO Patent Application WO/2011/007358, was submitted by Circ MedTech, Ltd, the company that currently markets the PrePex System.[3]
Promotion of Male Circumcision for HIV Prevention
PrePex was set to be introduced to Zimbabwe to aid their mass circumcision campaign.[4]
PlusNews reported on a study of the PrePex device, saying "a study of 40 men in Rwanda found one adverse event at removal of the device and a healing time of 17 days".[5]
Circ MedTech promotes the PrePex System as a means to "to dramatically reduce the HIV infection rate." Built in to the patent application is a statement touting male circumcision as an HIV prevention method.[6]
Use in Africa
The PrePex device will be tested in Africa starting 2013. The President's Emergency Plan for AIDS Relief will pay for PrePex circumcisions for about 2,500 men in Lesotho, Malawi, South Africa, Tanzania and Uganda. The Bill & Melinda Gates Foundation will pay for similar studies in Kenya, Mozambique, South Africa, Zambia and Zimbabwe. [7] The Rakai Health Sciences Program is currently unveiling it and testing it in Uganda, presented by Godfrey Kigozi, program chief investigator, who stated that "it was invented in the United States of America. We have so far tested it on about 50 clients but we need 250 more to confirm its acceptability but so far it has worked properly with those who used it"[8]. The PrePex was not invented in the United States[9].
External links
- PrePex™ website
- Circ MedTech
- PrePex patent
- AIDS Prevention inspires ways to make circumcision easier
References
- ↑
 Scientific American.
Scientific American. Rwanda Investigating Adult Male Circumcision sans Anesthesia
, Nature America, Inc.. Retrieved 30 August 2011. - ↑

Safety and efficacy validated. Device approved for use in Rwanda
, PrePex. Retrieved 30 August 2011. - ↑

Circumcision device and method for mass circumcision
, WIPO. Retrieved 30 August 2011. - ↑

Controversial UN program brings circumcision device to Zimbabwe
. Retrieved 21 June 2012. - ↑

HIV/AIDS: Adult male circumcision – new developments
. Retrieved 20 June 2012. - ↑

Why Male Circumcision?
, PrePex. Retrieved 30 August 2011. - ↑

Africa: Nonsurgical Circumcision Device Will Be Tested to Help Curb AIDS
, The New York Times. Retrieved 26 December 2012. - ↑

New safe male circumcision method unveile
, New Vision. Retrieved 26 December 2012. - ↑

Circumcision device and method for mass circumcision
, WIPO. Retrieved 30 August 2011.