Foreskin
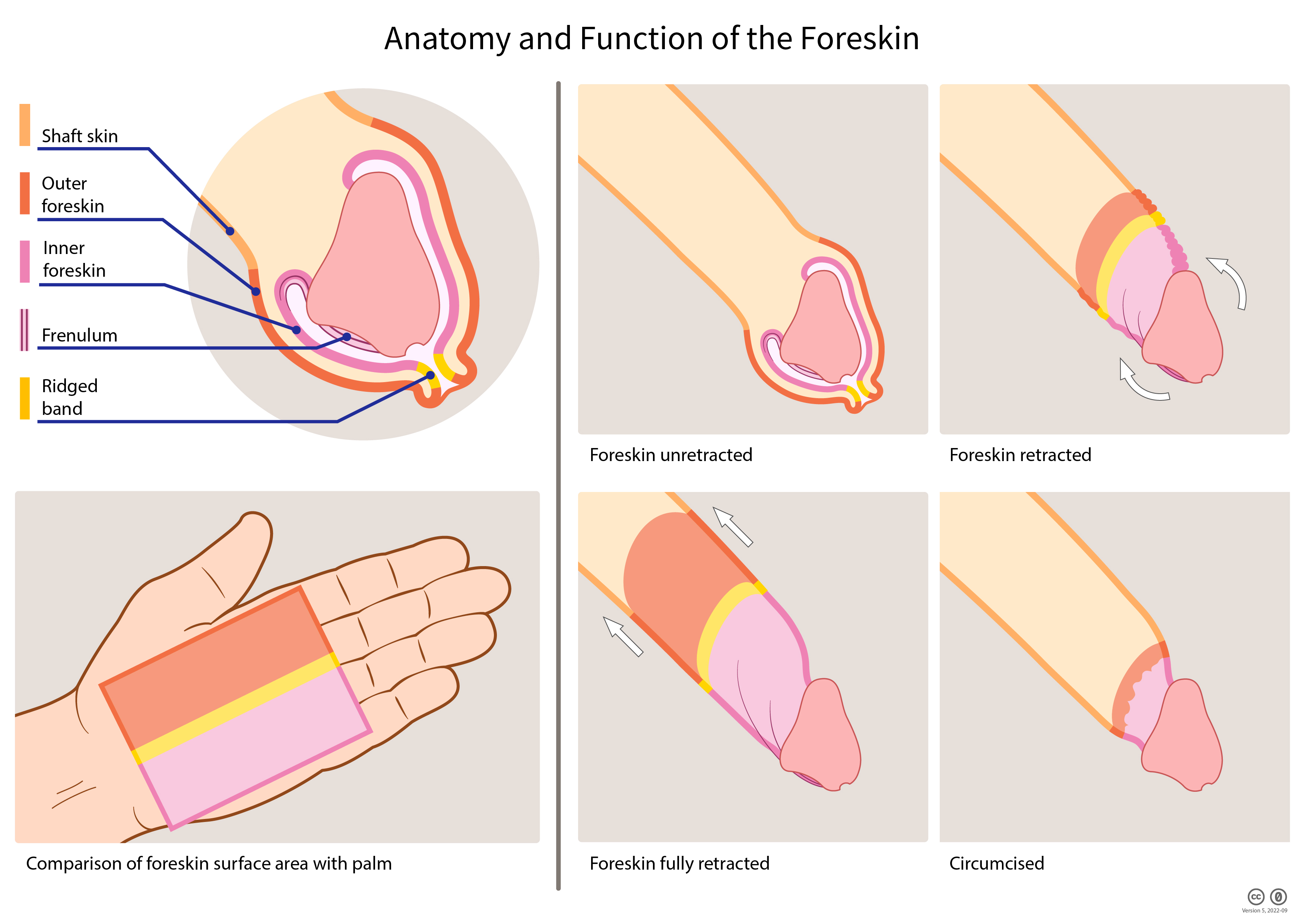
The foreskin or prepuce is the double-layered fold of smooth muscle tissue, blood vessels, neurons, skin, and mucous membrane part of the penis that covers and protects the glans penis and the urinary meatus.
The nature of the prepuce or foreskin, which is amputated and destroyed by circumcision, must be considered and fully understood in any discussion of male circumcision.[1]
Purpura et al. (2018) describe the foreskin as follows:
Few parts of the human anatomy can compare to the incredibly multifaceted nature of the human foreskin. At times dismissed as “just skin,” the adult foreskin is, in fact, a highly vascularized and densely innervated bilayer tissue, with a surface area of up to 90 cm, and potentially larger. On average, the foreskin accounts for 51% of the total length of the penile shaft skin and serves a multitude of functions. The tissue is highly dynamic and biomechanically functions like a roller bearing; during intercourse, the foreskin “unfolds” and glides as abrasive friction is reduced and lubricating fluids are retained. The sensitive foreskin is considered to be the primary erogenous zone of the male penis and is divided into four subsections: inner mucosa, ridged band, frenulum, and outer foreskin; each section contributes to a vast spectrum of sensory pleasure through the gliding action of the foreskin, which mechanically stretches and stimulates the densely packed corpuscular receptors. Specialized immunological properties should be noted by the presence of [[[Langerhans cells]] and other lytic materials, which defend against common microbes, and there is robust evidence supporting HIV protection. The glans and inner mucosa are physically protected against external irritation and contaminants while maintaining a healthy, moist surface. The foreskin is also immensely vascularized and acts as a conduit for essential blood vessels within the penis, such as supplying the glans via the frenular artery.[2]
The medical community usually omits information on the nature and functions of the foreskin from information provided to the public and to parents.
Contents
Structure
Foreskin in infancy and childhood
Baby boys are born with the foreskin fused with the glans penis by the balanopreputial lamina, a synechial membrane, which prevents retraction. In addition, the tip of the foreskin (acroposthion) is usually too narrow to allow retraction of the foreskin. The preputial cavity is closed by the synechia and cannot be infected.[3]
Forcible attempts to retract the foreskin result in injury to the boy, so should be avoided. The first person to retract the foreskin should be the boy himself.[4]
Thorvaldsen & Meyhoff (2005) conducted a survey of 4,000 boys in Denmark. They reported that the mean age of first foreskin retraction is 10.4 years in Denmark.[5]
Foreskin in adulthood
The foreskin (also known as the prepuce) is the double-layered fold of smooth muscle tissue, blood vessels, neurons, skin, and mucous membrane part of the penis that covers and protects the glans penis and the urinary meatus.[1] The adult foreskin measures about 3 inches by 5 inches or 15 square inches.[6] The two foreskin layers provide a skin reserve. When the penis becomes erect, the foreskin may wholly or partially unfold to provide the necessary skin to allow for expansion of the penis during erection.
The foreskin is the movable part of the penis. The foreskin is not attached to the underlying structure and is free to glide back and forth.[7] The gliding action reduces the friction of intercourse and helps to prevent abrasions, while conserving vaginal lubrication and moisture.[8] The foreskin is attached to the glans penis on the ventral side by the frenulum, which usually limits retraction to a widely variable degree. The ridged band emanates from the frenulum.[1]
The epithelium of the outer layer of the foreskin is true skin while the epithelium of the inner layer is mucosal membrane. There is a mucocutaneous boundary at the tip of the foreskin. The mucocutaneous junction is a specific erogenous zone.[9] Taylor et al. reported:
The vascular ridges of the `ridged band' and its Meissner's corpuscles firmly separate preputial epithelium from true skin and place preputial mucosa amongst other mucocutaneous mucosae. Winkelmann emphasized the structural and functional importance of junctional regions of the body and focused on mucocutaneous end-organs, or `genital corpuscles', of the glans penis and prepuce. Some of these end-organs resemble Krause end-bulbs; others resemble Meissner's corpuscles. … Meissner's corpuscles of the prepuce may be compared with similar nerve-endings in the finger-tips and lips, which respond in a fraction of a second to contact with light objects that bring about deformation of their capsules. … The prepuce provides a large and important platform for several nerves and nerve endings. The innervation of the outer skin of the prepuce is impressive; its sensitivity to light touch and pain are similar to that of the skin of the penis as a whole.[6]
The ridged band area is found at the mucocutaneous junction. The ridged band area is characterized by rete ridges with Meissner's corpuscles in the ridges.[6]
As with other neurologic structures such as the brain, the tip of the prepuce is richly supplied with blood by important vascular structures.[6] The prepuce serves as a conduit for several important veins.[3] The glans penis receives blood through the frenular artery.[10] The adult foreskin frequently has prominent visible veins.
The layer of dartos muscle is contained within the foreskin.[1] The dartos muscle keeps the foreskin snugly against the glans. The fibers of the dartos muscle form a whorl at the tip that functions as a sphincter. The sphincter opens to allow urine to flow out, but closes to protect the penis from foreign matter, contaminants, and pathogens.[7][11]
Natural foreskins are found in all lengths from very short to very long. Most fall near the center of the range.
The tapered tip of a longer foreskin that extends beyond the glans penis is known as the acroposthion. A very few of the longest foreskins will not remain retracted when released, but will spontaneously glide forward to recover the glans penis. Longer foreskins improve protection and gliding action and do not usually create an issue. There is no recognized definition of an excessively long foreskin.[12]
Physiological functions
Antique research by Alfred Kinsey (1948)[13] and Masters & Johnson (1966)[14] misled medical science into believing that the foreskin had no function. This meant the foreskin could be excised without doing harm. In actuality, the foreskin has many functions, so its amputation does great harm.
Protective functions
The foreskin provides physical protection to the glans penis and inner mucosa, supplying moisture and natural oils by transudation, while protecting the mucosal tissue from pathogens, pollutants, friction, injury, keratinization, de-sensitization, and drying out. The foreskin protects the glans penis and meatus of infant boys from abrasion and irritation from ammoniacal diapers.[15] [16]
In the absence of the foreskin about ten to twenty percent of boys suffer urethral stricture (meatal stenosis) requiring further treatment.[17] Frisch & Simonsen (2016) reported the incidence of meatal stenosis to be 3.7 times higher in circumcised boys.[17]
The foreskin continues to protect the glans penis throughout life, keeping it smooth, glossy, moist, sensitive, and free from keratinization and loss of sensation.
Immunological functions
Fleiss et al. (1998) have listed numerous immunological functions of the foreskin that help to protect the human body against infection. The foreskin maintains the moistness of the preputial mucosa and the glans penis by transudation. The sub-preputial moisture contains cathepsin B, chymotrypsin, neutrophil elastase, cytokines, and lysozyme, which has the capacity to desttroy the cell walls of bacteria. The preputial muscles keep the tip of the foreskin closed to keep out pathogens.[3]
The foreskin is highly vascularized. The high rate of blood flow helps to prevent infection.[3]
Although claims have been made that the presence of the foreskin increases the risk of infection with human immune deficiency virus (HIV), that is not correct. The claims are based on the reported findings of three randomized clinical trials (RCTs) that were carried out more than a decade ago in Africa. Boyle & Hill (2011) have shown these RCTs to be have significant methodological flaws and statistical errors that render their claims invalid.[18]
Fleiss et al. noted the presence of Langerhans cells in the prepuce.[3] De Witte et al. (2007) have discovered that Langerhans cells produce Langerin, which is a "natural barrier to HIV infection".[19]
For more information, see Immunological and protective function of the foreskin.
Sexual functions
The foreskin is a sexual organ. It provides both mechanical and erogenous functions in sexual intercourse, as well as pheromones.[3] Winkelmann (1959) classified the foreskin as specific erogenous tissue.[9] When the penis becomes erect, the foreskin unfolds to provide the skin necessary to allow the penis to expand to full size and length.
The gliding action provides stimulation and facilitates intromission.[8][20] [21] During the thrusting of sexual congress, the gliding action reduces abrasions and irritation in the female partner and avoids problems with vaginal dryness.[8][20]
Sensory functions
Nature designed the foreskin to be an erogenous sensory organ. The foreskin has a layer of muscle called the dartos muscle sheath that provides the foreskin with elasticity, flexibility, and stretchiness, which allows full stimulation of the nerves in the ridged band that sense movement and stretching to provide foreskin sensitivity. There is a mucocutaneous region at the tip of the foreskin where outer skin changes to inner mucosa. Winkelmann (1959) identified the foreskin as a specific erogenous zone (meaning an area of acute erogenous sensation). Winklemann reported rete ridges in the foreskin with nerves set close to the surface with closely set networks.[9]
Lakshamanan & Prakash (1980) report the "prepuce covers the glans completely and snugly like a hosiered material and continues to do so through the entire span of life of the male", which they explain as being caused by the smooth muscle fibers within the prepuce. The prepuce is free to glide back and forth. When it does, it must stretch to go over the glans penis.[7]
Taylor et al. (1996) carried out a histological study of the foreskin. (Histology is the microscopic examination of cells and tissues.) Taylor et al. found an area of rete ridges just inside the tip of the foreskin that he called the ridged band. The ridges were found to have nerve endings at the top of the ridges. They report that the ridged band area moves to the shaft of the penis when the penis becomes erect where the nerves are subject to stimulation. They stated:
The prepuce provides a large and important platform for several nerves and nerve endings. The innervation of the outer skin of the prepuce is impressive; its sensitivity to light touch and pain are similar to that of the skin of the penis as a whole.[6]
Sorrells et al. (2007) conducted a fine-touch study of the penis of both circumcised and intact men. Sorrells et al. concluded:
In conclusion, circumcision removes the most sensitive parts of the penis and decreases the fine-touch pressure sensitivity of glans penis. The most sensitive regions in the uncircumcised penis are those parts ablated by circumcision. When compared to the most sensitive area of the circumcised penis, several locations on the uncircumcised penis (the rim of the preputial orifice, dorsal and ventral, the frenulum near the ridged band, and the frenulum at the muco-cutaneous junction) that are missing from the circumcised penis were significantly more sensitive.[22]
Bronselaer et al. (2013) surveyed a large group of intact and circumcised men in Belgium. They reported:
Of the men in Group A [intact males], 90.6% rated the sexual pleasure provided when the foreskin was stimulated by themselves or their partners from ‘mild’ to ‘very strong’ and 61.9% the respective orgasm from ‘mild’ to ‘very strong’.
For the glans penis, men in Group B [circumcised males] reported significantly less sexual pleasure than men in Group A at the dorsal side (P ≤ 0.001), and the lateral (P ≤ 0.001) and ventral sides (P = 0.02). Orgasm was less intense in Group B at the dorsal side (P = 0.006) and at the lateral sides (P = 0.02). Group B required more effort in achieving orgasm at the lateral sides (P = 0.04). Furthermore, a larger percentage of men in this group reported numbness at the dorsal, lateral, and ventral sides (all P ≤ 0.001), as well as unusual sensations (burning, prickling, itching, or tingling) at the lateral sides (P = 0.02) and at the ventral side (P = 0.003) of the glans.[23]
García-Mesa et al. (2021) reported the foreskin has an abundance of Meissner's corpuscles which provide pleasant sexual sensation when mechanically stimulated by motion of the foreskin.[24]
Sexual behavior
Laumann et al. (1997) reported that men who lack a foreskin "engage in a somewhat more elaborated set of sexual practices than do men who are not circumcised. For each of the practices examined, lifetime experience of various forms of oral and anal sex and masturbation frequency in the past year, circumcised men engaged in these behaviors at greater rates. The difference between circumcised men was greatest for masturbation — ironically, a practice that circumcision was once thought to limit. A total of 47% of circumcised men reported masturbating at least once a month vs 34% for their uncircumcised peers."[25] Dave et al. (2003) reported that men without foreskins "were more likely to report having had homosexual partner(s) (7.5% v 5.3%, p =0.012) and partners from abroad (19.7% v 13.1%, p...0.001)."[26]
Cold & Taylor (1999) commented, "The increased frequency of masturbation, anal intercourse and fellatio reported by circumcised men in the USA may possibly be due to the sensory imbalance caused by circumcision. Clearly, amputation of the prepuce causes changes in sexual behaviour in human males and females."[1]
Frisch et al. (2011) surveyed the sexual function and behavior of a large group of intact and circumcised men in Denmark. They reported that "circumcised men were more likely (38%) than uncircumcised men (28%) to report ≥10 sex partners."[27]
The role of the foreskin in heterosexual relations
The knowledge of the important of the intact penis in heterosexual relations long has been known to the Jews who give us our earliest evidence of the value of the foreskin to the female partner.
Moses Maimonides, a Jewish rabbi and physician, stated in the 12th century:
| “ | It is hard for a woman with whom an uncircumcised man has had sexual intercourse to separate from him. – Moses Maimonides[28] |
According to the Rabbi Isaac ben Yedaiah, who lived in Southern France in the late 13th century:
She too will court the man who is uncircumcised in the flesh and lie against his breast with great passion, for he thrusts inside her a long time because of his foreskin, which is a barrier against ejaculation in intercourse. Thus she feels pleasure and reaches an orgasm first. When an uncircumcised man sleeps with her, and then resolves to return to his home, she brazenly grabs him, holding on to his genitals and says to him, "Come back, make love to me." This is because of the pleasure that she finds in intercourse with him, from the sinews of his testicles—sinew of iron—and from his ejaculation—that of a horse which he shoots like an arrow into her womb. They are united without separating, and he makes love twice and three times in one night, yet the appetite is not filled.[29]
As previously reported, the foreskin reduces the force required for penetration of the female partner's vagina by as much as ninety percent.[21] The gliding action of the foreskin reduces friction and abrasion, while conserving vaginal lubrication.[8]
In a first of its kind, O'Hara & O'Hara (1999) carried out a retrospective survey of 138 women with experience of both intact and circumcised partners. The women overwhelmingly concurred that the mechanics of coitus was different for the two groups of men. Of the women, 73% reported that circumcised men tend to thrust harder and deeper, using elongated strokes, while unaltered men by comparison tended to thrust more gently, to have shorter thrusts, and tended to be in contact with the mons pubis and clitoris more, according to 71% of the respondents. Women with intact partners had a higher rate of orgasms than women with circumcised partners. O'Hara & O'Hara concluded:
Clearly, the anatomically complete penis offers a more rewarding experience for the female partner during coitus. While this study has some obvious methodological flaws, all the differences cannot be attributed to them. It is important that these findings be confirmed by a prospective study of a randomly selected population of women with experience with both types of men. It would be useful to examine the role of the foreskin in other sexual activities. Because these findings are of interest, the negative effect of circumcision on the sexual enjoyment of the female partner needs to be part of any discussions providing 'informed consent' before circumcision.[30]
Solinis & Yiannaki (2007) concluded; "[t]here was a decrease in couple’s sexual life after circumcision indicating that adult circumcision adversely affects sexual function in many men or/and their partners, possibly because of complications of surgery and loss of nerve endings."[31]
Frisch et al. (2011) surveyed a very large group of men and women in Denmark. They concluded:
"Circumcision was associated with frequent orgasm difficulties in Danish men and with a range of frequent sexual difficulties in women, notably orgasm difficulties, dyspareunia and a sense of incomplete sexual needs fulfilment."[27]
See also
- Acroposthion
- Foreskin Care for Boys
- Foreskin in Motion
- Foreskin sensitivity
- Frenulum
- Immunological and protective function of the foreskin
- Retraction of the foreskin
- Ridged band
- The Foreskin and Why You Should Keep It (book)
External links
 Wallerstein, Edward: When Your Baby Boy is Not Circumcised (Four-page pamphlet), Pennypress (Seattle). (February 1982). Retrieved 30 May 2021.
Wallerstein, Edward: When Your Baby Boy is Not Circumcised (Four-page pamphlet), Pennypress (Seattle). (February 1982). Retrieved 30 May 2021. Fleiss P. The foreskin is necessary. Mothering. January 1997; : 36-45. Retrieved 29 June 2020.
Fleiss P. The foreskin is necessary. Mothering. January 1997; : 36-45. Retrieved 29 June 2020. Erickson, John (1999).
Erickson, John (1999). 33 Photographs of the foreskin (Non-Pornographic)
, Foreskin. Retrieved 25 October 2019. Hill, George (14 June 2008).
Hill, George (14 June 2008). Ch. 2: The Prepuce (DOC Genital Integrity Statement)
, Research Hub, Doctors Opposing Circumcision (D.O.C.). Retrieved 15 December 2019. Young, Hugh (2010).
Young, Hugh (2010). NSFW Video: How the foreskin works
, Circumstitions. Retrieved 25 October 2019. Quine, Spoony (8 May 2013).
Quine, Spoony (8 May 2013). The foreskin: Why is it such a secret in North America?
, Mad Science Writer. Retrieved 19 April 2020. Harryman, Gary L. (22 November 2013).
Harryman, Gary L. (22 November 2013). The Foreskin Advantage
, NOHARMM. Retrieved 2 September 2021. Helard, Lou (1 August 2014).
Helard, Lou (1 August 2014). Functions of the Foreskin
, Intact Australia. Retrieved 29 May 2020. Goldman, Ronald.
Goldman, Ronald. Functions of the Foreskin
, circumcision.org, Circumcision Resource Center. Retrieved 1 July 2020. (July 2016).
(July 2016). The prepuce
, Doctors Opposing Circumccision. Retrieved 16 October 2019.
Quote:To provide informed, respectful care to males with intact genitals, and to fully understand the harms of circumcision, health professionals must have a thorough grasp of the anatomy, development, and functions of the foreskin (also known as the prepuce).
 Harryman, Gary L.
Harryman, Gary L. The Foreskin Advantage
, NOHARMM, NORM/Southern California. Retrieved 30 June 2020.
Functions of the foreskin
. Retrieved 30 May 2021. Taylor, John R. (1996).
Taylor, John R. (1996). Ridged band: Specialized tissue of the penis
, CIRP. Retrieved 21 July 2021. Chapin, Georganne (13 May 2016).
Chapin, Georganne (13 May 2016). Promoting the Prepuce
, Intact America. Retrieved 2 April 2021. Troy, Louise (24 May 2022).
Troy, Louise (24 May 2022). Male Foreskin is Functional – 5 Amazing Facts
, 15 Square. Retrieved 24 May 2022.
Quote:The foreskin is the primary fine-touch sensory tissue of the penis.
References
- ↑ a b c d e
 Cold CJ, Taylor JR. The prepuce. BJU Int. January 1999; 83, Suppl. 1: 34-44. PMID. DOI. Retrieved 8 July 2021.
Cold CJ, Taylor JR. The prepuce. BJU Int. January 1999; 83, Suppl. 1: 34-44. PMID. DOI. Retrieved 8 July 2021.
- ↑
 Purpura V, Bondioli E, Cunningham EC, et al. The development of a decellularized extracellular matrix–based biomaterial scaffold derived from human foreskin for the purpose of foreskin reconstruction in circumcised males. J Tissue Eng. 22 December 2018; PMID. PMC. DOI. Retrieved 9 February 2020.
Purpura V, Bondioli E, Cunningham EC, et al. The development of a decellularized extracellular matrix–based biomaterial scaffold derived from human foreskin for the purpose of foreskin reconstruction in circumcised males. J Tissue Eng. 22 December 2018; PMID. PMC. DOI. Retrieved 9 February 2020.
- ↑ a b c d e f
 Fleiss P, Hodges F, Van Howe RS. Immunological functions of the human prepuce. Sex Trans Infect. October 1998; 74(5): 364-67. PMID. PMC. DOI. Retrieved 14 January 2022.
Fleiss P, Hodges F, Van Howe RS. Immunological functions of the human prepuce. Sex Trans Infect. October 1998; 74(5): 364-67. PMID. PMC. DOI. Retrieved 14 January 2022.
- ↑
 Wright JE. Further to the "Further Fate of the Foreskin". Med J Aust. 7 February 1994; 160: 134-135. PMID. Retrieved 16 May 2020.
Wright JE. Further to the "Further Fate of the Foreskin". Med J Aust. 7 February 1994; 160: 134-135. PMID. Retrieved 16 May 2020.
- ↑
 Thorvaldsen MA, Meyhoff H. Patologisk eller fysiologisk fimose? [Pathological or physiological phimosis?] (Danish). Ugeskr Læger. 2005; 167(17): 1858-1862. Retrieved 14 November 2019.
Thorvaldsen MA, Meyhoff H. Patologisk eller fysiologisk fimose? [Pathological or physiological phimosis?] (Danish). Ugeskr Læger. 2005; 167(17): 1858-1862. Retrieved 14 November 2019.
- ↑ a b c d e
 Taylor JR, Lockwood AP, Taylor AJ. The prepuce: specialized mucosa of the penis and its loss to circumcision. Br J Urol. 1996; 77: 291-5. PMID. DOI. Retrieved 23 September 2019.
Taylor JR, Lockwood AP, Taylor AJ. The prepuce: specialized mucosa of the penis and its loss to circumcision. Br J Urol. 1996; 77: 291-5. PMID. DOI. Retrieved 23 September 2019.
- ↑ a b c
 Lakshmanan S, Prakash S. Human prepuce: some aspects of structure and function. Indian J Surg. 1980; 44: 134-7.
Lakshmanan S, Prakash S. Human prepuce: some aspects of structure and function. Indian J Surg. 1980; 44: 134-7.
- ↑ a b c d
 Warren J, Bigelow J. The case against circumcision. Brit J Sex Med. September 1994; Retrieved 28 October 2019.
Warren J, Bigelow J. The case against circumcision. Brit J Sex Med. September 1994; Retrieved 28 October 2019.
- ↑ a b c
 Winkelmann RK. The erogenous zones: their nerve supply and significance. Mayo Clin Proc. 21 January 1959; 34(3): 39-47. PMID. Retrieved 14 October 2019.
Winkelmann RK. The erogenous zones: their nerve supply and significance. Mayo Clin Proc. 21 January 1959; 34(3): 39-47. PMID. Retrieved 14 October 2019.
- ↑
 Persad R, Sharma S, McTavish J, et al. Clinical presentation and pathophysiology of meatal stenosis following circumcision. Brit J Urol. 1995; 75(1): 91-3. PMID. DOI. Retrieved 15 October 2019.
Persad R, Sharma S, McTavish J, et al. Clinical presentation and pathophysiology of meatal stenosis following circumcision. Brit J Urol. 1995; 75(1): 91-3. PMID. DOI. Retrieved 15 October 2019.
- ↑
 Jefferson. The peripenic muscle; some observations on the anatomy of phimosis. Surgery, Gynecology, and Obstetrics (Chicago). 1916; 23(2): 177-81. Retrieved 14 October 2019.
Jefferson. The peripenic muscle; some observations on the anatomy of phimosis. Surgery, Gynecology, and Obstetrics (Chicago). 1916; 23(2): 177-81. Retrieved 14 October 2019.
- ↑
 (2022).
(2022). The ‘Tip’ At The End
, Acroposthion. Retrieved 21 June 2022. - ↑
 Kinsey, Alfred C. (1948): Sexual behavior in the human male. Retrieved 16 June 2022.
Kinsey, Alfred C. (1948): Sexual behavior in the human male. Retrieved 16 June 2022.
- ↑
 Masters, William (1966): Human sexual response. ISBN 978-0-553-20429-2. Retrieved 16 June 2022.
Masters, William (1966): Human sexual response. ISBN 978-0-553-20429-2. Retrieved 16 June 2022.
- ↑
 Gairdner DMT. The fate of the foreskin: a study of circumcision. British Medical Journal. 1949; 2(4642): 1433-7. PMID. PMC. DOI. Retrieved 28 October 2019.
Gairdner DMT. The fate of the foreskin: a study of circumcision. British Medical Journal. 1949; 2(4642): 1433-7. PMID. PMC. DOI. Retrieved 28 October 2019.
- ↑ a b
 Frisch M, Simonsen J. Cultural background, non-therapeutic circumcision and the risk of meatal stenosis and other urethral stricture disease: Two nationwide register-based cohort studies in Denmark 1977-2013. The Surgeon. 1 April 2016; 16(2): 107-18. PMID. DOI. Retrieved 23 October 2019.
Frisch M, Simonsen J. Cultural background, non-therapeutic circumcision and the risk of meatal stenosis and other urethral stricture disease: Two nationwide register-based cohort studies in Denmark 1977-2013. The Surgeon. 1 April 2016; 16(2): 107-18. PMID. DOI. Retrieved 23 October 2019.
- ↑
 Boyle GJ, Hill G. Sub-Saharan African randomised clinical trials into male circumcision and HIV transmission: Methodological, ethical and legal concerns
Boyle GJ, Hill G. Sub-Saharan African randomised clinical trials into male circumcision and HIV transmission: Methodological, ethical and legal concerns  . Thompson Reuter. December 2011; 19(2): 316-34. PMID. Retrieved 30 December 2020.
. Thompson Reuter. December 2011; 19(2): 316-34. PMID. Retrieved 30 December 2020.
- ↑
 de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TBH. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature Medicine. 4 March 2007; PMID. DOI. Retrieved 28 June 2011.
de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TBH. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nature Medicine. 4 March 2007; PMID. DOI. Retrieved 28 June 2011.
- ↑ a b
 Morgan WKC. The rape of the phallus. JAMA. 1965; 193: 123-4. PMID. DOI. Retrieved 15 October 2019.
Morgan WKC. The rape of the phallus. JAMA. 1965; 193: 123-4. PMID. DOI. Retrieved 15 October 2019.
- ↑ a b
 Taves D. The intromission function of the foreskin. Med Hypotheses. 2002; 59(2): 180. PMID. DOI. Retrieved 15 October 2019.
Taves D. The intromission function of the foreskin. Med Hypotheses. 2002; 59(2): 180. PMID. DOI. Retrieved 15 October 2019.
- ↑
 Sorrells ML, Snyder JL, Reiss MD, Eden C, Milos MF, Wilcox N, Van Howe RS. Fine‐touch pressure thresholds in the adult penis. BJUI. 19 March 2007; 99(4): 864-9. PMID. DOI. Retrieved 10 January 2021.
Sorrells ML, Snyder JL, Reiss MD, Eden C, Milos MF, Wilcox N, Van Howe RS. Fine‐touch pressure thresholds in the adult penis. BJUI. 19 March 2007; 99(4): 864-9. PMID. DOI. Retrieved 10 January 2021.
- ↑
 Bronselaer G, Schoeber JM, Meyer-Bahlburg HM, T'Sjoen G, Vlietinck R, Hoebeke PB. Male circumcision decreases penile sensitivity as measured in a large cohort. BJU Int. May 2013; 111(5): 820-7. DOI. Retrieved 23 October 2019.
Bronselaer G, Schoeber JM, Meyer-Bahlburg HM, T'Sjoen G, Vlietinck R, Hoebeke PB. Male circumcision decreases penile sensitivity as measured in a large cohort. BJU Int. May 2013; 111(5): 820-7. DOI. Retrieved 23 October 2019.
- ↑
 García-Mesa, Yolanda, García-Piqueras, Jorge, Cobo, Ramón, Martín-Cruces, José, Suazo, Iván, García-Suárez, Olivia, Feito, Jorge. Sensory innervation of the human male prepuce: Meissner's corpuscles predominate. Journal of Anatomy. October 2021; 239(4): 892-902. PMID. PMC. DOI. Retrieved 15 November 2021.
García-Mesa, Yolanda, García-Piqueras, Jorge, Cobo, Ramón, Martín-Cruces, José, Suazo, Iván, García-Suárez, Olivia, Feito, Jorge. Sensory innervation of the human male prepuce: Meissner's corpuscles predominate. Journal of Anatomy. October 2021; 239(4): 892-902. PMID. PMC. DOI. Retrieved 15 November 2021.
- ↑
 Laumann EO, Masi CM, Zuckerman CW. Circumcision in the United States. JAMA. 2 April 1997; 277(13): 1052-7. PMID. Retrieved 23 October 2019.
Laumann EO, Masi CM, Zuckerman CW. Circumcision in the United States. JAMA. 2 April 1997; 277(13): 1052-7. PMID. Retrieved 23 October 2019.
- ↑
 Dave SS, Johnson AM, Fenton KA, Mercer CH, Erens B, Wellings K. Male circumcision in Britain: findings from a national probability sample survey. Sex Trans Infect. December 2003; 79: 499-500. PMID. PMC. DOI. Retrieved 29 October 2019.
Dave SS, Johnson AM, Fenton KA, Mercer CH, Erens B, Wellings K. Male circumcision in Britain: findings from a national probability sample survey. Sex Trans Infect. December 2003; 79: 499-500. PMID. PMC. DOI. Retrieved 29 October 2019.
- ↑ a b
 Frisch M, Lindholm M, Grønbæk M. Male circumcision and sexual function in men and women: a survey-based, cross-sectional study in Denmark. Int J Epidemiol. 14 June 2011; 40(5): 1367-81. PMID. DOI. Retrieved 26 September 2021.
Frisch M, Lindholm M, Grønbæk M. Male circumcision and sexual function in men and women: a survey-based, cross-sectional study in Denmark. Int J Epidemiol. 14 June 2011; 40(5): 1367-81. PMID. DOI. Retrieved 26 September 2021.
- ↑
 Maimonides M (1963): The Guide of the Perplexed. P. 609. Retrieved 11 October 2019.
Maimonides M (1963): The Guide of the Perplexed. P. 609. Retrieved 11 October 2019.
- ↑
 Saperstein M (1980): Decoding the Rabbis: A Thirteenth-Century Commentary on the Aggadah. Retrieved 22 October 2019.
Saperstein M (1980): Decoding the Rabbis: A Thirteenth-Century Commentary on the Aggadah. Retrieved 22 October 2019.
- ↑
 O'Hara K, O'Hara J, et al. The effect of male circumcision on the sexual enjoyment of the female partner. BJU Int. 1999; 83 Suppl 1: 79-84. PMID. DOI. Retrieved 22 October 2019.
O'Hara K, O'Hara J, et al. The effect of male circumcision on the sexual enjoyment of the female partner. BJU Int. 1999; 83 Suppl 1: 79-84. PMID. DOI. Retrieved 22 October 2019.
- ↑
 Solinis, Yiannaki. Does circumcision improve couple's sex life?. Journal of Men's Health and Gender. September 2007; 4(3): 361. Retrieved 23 October 2019.
Solinis, Yiannaki. Does circumcision improve couple's sex life?. Journal of Men's Health and Gender. September 2007; 4(3): 361. Retrieved 23 October 2019.